A lawsuit claims that 12 Indonesian families have filed claims against the government and the companies allegedly responsible for supplying the tainted cough syrups after relatives therein passed away or suffered injuries.
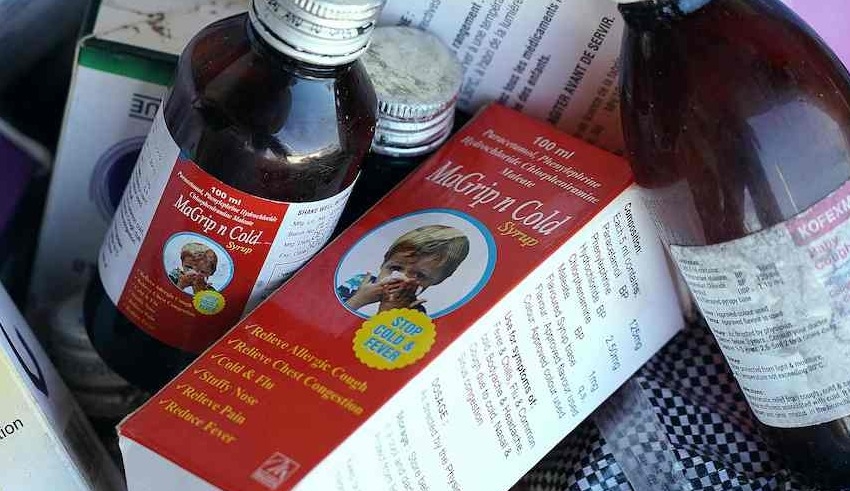
Since August, there has been an increase in acute kidney injury cases in the Southeast Asian nation, and the deaths of at least 199 people—mostly children—have prompted an investigation and a sales ban on some liquid medications.
The health ministry, the nation’s food and drug agency, and seven businesses are the targets of the class action lawsuit for selling syrups that contained too much of two substances used in antifreeze and other industrial products.
According to Awan Puryadi, a legal representative for the victims’ families, they took action because the government did nothing to stop the sale of dangerous medicines and the ensuing deaths or injuries of children.
Keep Reading
Puryadi commented on the case on December 1: “No one has taken responsibility, and they are quite upset with the current scenario.” The complaint was filed on November 18.
“We demand that the BPOM (food and drug agency), pharmacies, drug suppliers, and health ministry all accept responsibility.”
According to the attorney, families are requesting compensation of approximately 2 billion rupiah (US$127,049) for each person murdered and approximately 1 billion rupiah (US$63,524) for each person injured.
He said that additional families could join at a later date. According to the lawsuit, two families are bringing legal action on behalf of a total of 12 families.
At least three businesses that had their licenses to manufacture syrup medications temporarily frozen are the subject of a police inquiry.
The Gambia in West Africa reported 70 child fatalities in October that are thought to have been brought on by imported syrup medications.
The World Health Organization warned that the deaths could be related to the “unacceptable amount” of diethylene glycol and ethylene glycol found in four cough syrups made in India.


























