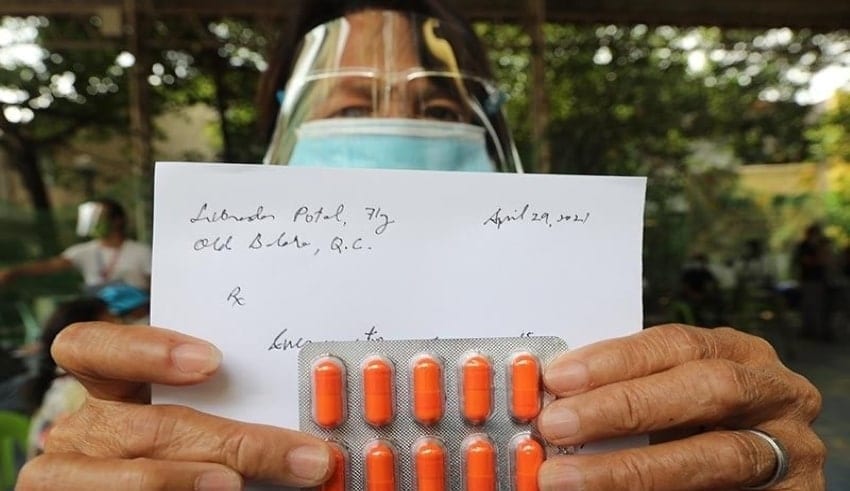
The Food and Drug Administration should investigate the medicines that did not have specialists’ data given to beneficiaries of the counter parasitic medication ivermectin around there. At the supposed storeroom coordinated by two legislators regardless of admonitions from clinical specialists, specialists gave recipients solutions for ivermectin. Yet, the solutions, composed on pieces of paper, didn’t contain their names, permit numbers and expert assessment receipt numbers.
All solutions should contain the name of the prescriber, office address, proficient enrollment number, proficient duty receipt number. They ought to follow this. They can’t give token solutions which was highlighted very well to remind the public.
Related Posts
In a different preparation, Health Undersecretary Maria Rosario Vergeire said such subtleties are needed as commanded by the Consumer Act of the Philippines 1991, Generics Act of 1988, and the FDA Act of 2009.
In an articulation gave Thursday evening, the DOH said it will embrace the reports of invalid remedies to the Professional Regulation Commission to research the veracity of the reports and force authorizes as considered significant dependent on existing laws.
While he said the FDA should examine the solutions given to recipients, Duque said there might be nothing illicit about the move of Deputy Speaker Rodante Marcoleta (Sagip party-rundown) and Rep. Michael Defensor (Anakalusugan party-lean) to part with ivermectin on the off chance that they followed the exclusions set by the FDA.
Meanwhile, It was shared that it’s just emergency clinics with sympathetic unique grants can give ivermectin as an off-mark COVID-19 treatment. Be that as it may, the wellbeing official kept up Friday there is insufficient proof to help the utilization of ivermectin to forestall or treat COVID-19.
The contrasting perspectives on the DOH and the FDA on whether the medication can be conveyed to the general population provoked the Quezon City Health Department to say the two offices should think of an unmistakable remain on the utilization of ivermectin.
Morever, Vergeire additionally said there are two “pathways” on any medication related choices: administrative and clinical. Administrative pathway falls under the FDA, while the clinical pathway is taken by the DOH and clinic
































