(C) Flickr, Manatee County Government
The Indonesian Food and Drug Monitoring Agency approved a COVID-19 vaccine produced for emergency use by China’s Sinovac Biotech, the chairwoman of the office, Penny K. Lukito.
The approval for emergency use was given after a thorough review of the interim results of Sinovac’s late clinical trials in Indonesia, Brazil, and Turkey of the vaccine. The results of Sinovac and state-owned vaccine manufacturer BioFarma’s trials conducted in Bandung showed that CoronaVac was 65.3 percent successful, effectively clearing the minimum standard set by World Health.
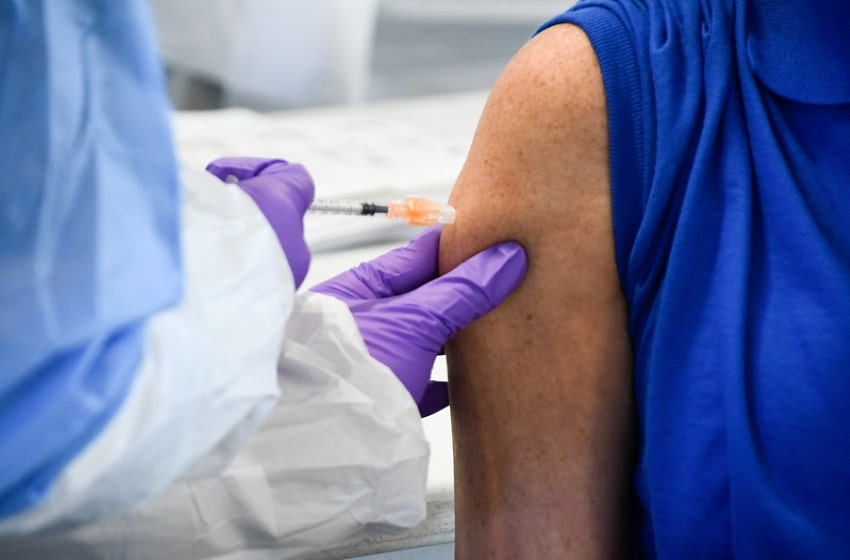
In view of this, Lukito shared to the press that BPOM would closely track the possible side effects that will occur during the first inoculation on Wednesday after approval. The Indonesian government will initiate its vaccine campaign, with the first COVID-19 vaccine shot being taken by President Joko Widodo, and the vaccination push scheduled to continue in many regions around the world on 14-15 January.
True enough, Jokowi is due to take his first injection of the COVID-19 vaccine today The process for inoculating the president has been planned by the Presidential Secretariat and the Health Ministry which will then be aired by live streaming.
The vaccine was actually tested to be 78 percent effective in Brazil, while the test result in Turkey was found to be 91 percent effective, Lukito revealed. The chairwoman also spoke of her office having met with the National Drug Review Committee, epidemiologists, and the Indonesian Immunization Technical Advisory Group (ITAGI) to share and peer-review the test results of the vaccine.
Prior to making the EUA decision, the Department had taken into account the findings of clinical vaccine studies performed in Indonesia, Brazil and Turkey that showed protection and effectiveness in the prevention of infection with COVID-19. The vaccine also complied with the recommendations of the World Health Organisation (WHO) with a minimum potency of 50 percent to secure a license.
Morever, The clinical test conducted by state-run vaccine producers Bio Farma and Sinovac in Bandung, West Java, found the vaccine to be 65.3 percent effective, while clinical test findings in Turkey and Brazil revealed that the vaccine was 91 percent and 78 percent effective which made the countries inoculate this vaccine.
OpenAI updated ChatGPT-4o to include its best text-to-image tools so free users can generate Studio Ghibli artwork by giving basic…
The stepping down of Piyush Gupta from the post of CEO of DBS Bank came after 15 years of leading…
The Delhi Directorate of Education releases 2025-26 marks for year-end tests in school levels 6 through 11. Online test data…
Singapore will further cement its status as an important basketball destination when it hosts three FIBA 3x3 events in 2026…
Jewel Section E, directed by Theodore Boborol and starring Ashtine Olviga as Jay-Jay Mariano, Andres Muhlach as Mark Keifer Watson,…
Cebu Pacific celebrates the delivery of its very first aircraft for 2025, the 459-seat Airbus A330neo, delivered at Ninoy Aquino…
This website uses cookies.
Read More