(C) Gadjah Mada University
Last updated on May 6th, 2021 at 10:24 am
Covid-19 positive patients in Indonesia continue to grow. Based on data from the Covid-19 Handling Task Force, there were 2,657 new positive cases, so that until Thursday (9/7), a total of 70,736 cases was recorded.
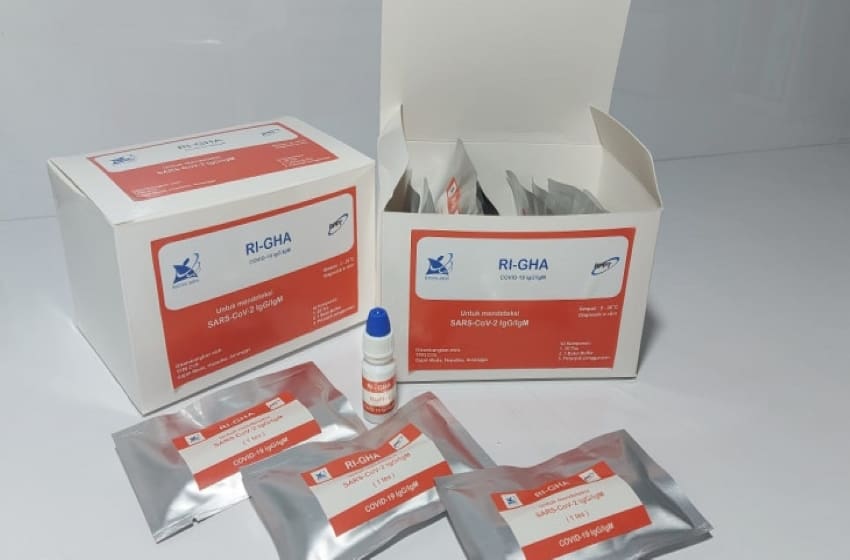
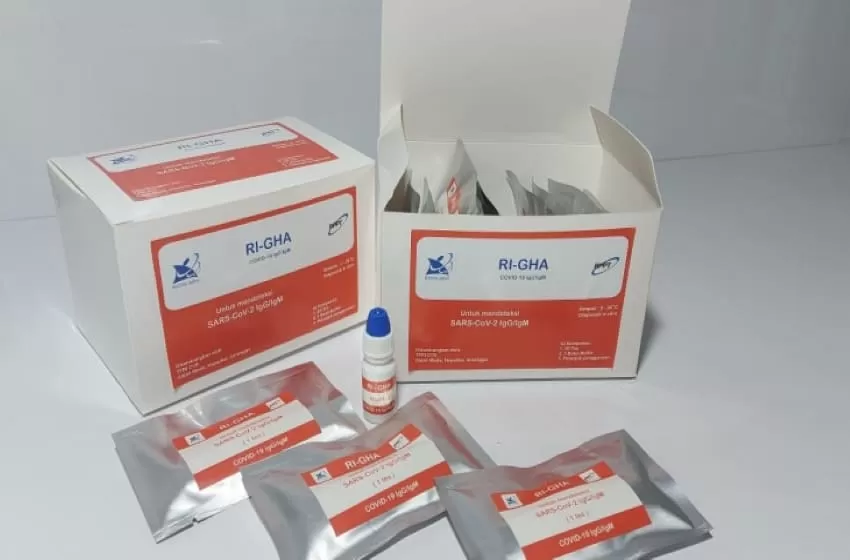
In an effort to handle Covid-19 across the country, the government launched a domestic rapid test tool called the RI-GHA Covid-19.
Minister of Research and Technology/Head of the National Research and Innovation Agency (Menristek/Head of BRIN) Bambang P.S Brodjonegoro said this tool was a collaboration with the Agency for Application and Assessment of Technology (BPPT), Gadjah Mada University, Airlangga University, Bandung Institute of Technology, Mataram University, and company PT Hepatika Mataram.
The government targets to produce 200 thousand rapid test tools in July and 400 thousand in August 2020.
Head of BPPT Hammam Riza said the rapid test equipment of domestic production is superior in quality and price compared to imported products. One unit of rapid test equipment made in the country costs Rp. 75,000.
“We believe that this rapid test product in the country is able to compete in the market and at lower prices. We must be prepared to compete in price with the same quality. It has to. If not, I am worried that we will continue to be crushed by imported products, “said Coordinating Minister for PMK Muhadjir Effendy in Jakarta, Thursday, July 9.
According to him, the supply of domestic products was in accordance with President Joko Widodo’s direction. Namely, the existence of regulations relating to government spending is simplified. In fact, government spending must also prioritize domestic products. Because this is considered to be able to spur economic growth in Indonesia.
Muhadjir greatly appreciated the findings of the country’s children in the form of the rapid test tool. The tool is staying able to be a solution for meeting the needs of medical devices that have been very urgent in Indonesia.
The same thing was said by Bambang that said the rapid test is one of the important elements in handling COVID-19. This rapid test tool has been tested for sensitivity (98 percent) and specificity (96 percent) through laboratory tests on Indonesians. This test tool is quite flexible because it is able to detect people without symptoms, people in monitoring, patient under surveillance, and post-infection
May is one of the crucial financial months in a year, if you have any important transactions or any official…
If you happen to breathe K-drama, then your 'May 2025' will most likely be well-rendered into a month! Romantic sagas,…
Since yesterday, May 2, 2023, at the Mall of Asia Arena in Pasay, Ahtisa Manalo has demonstrated her brilliance by…
“you’re nothing but a trying hard copycat” Character- Lavinia Arguelles Film- Bituing Walang Ningning (1985) Context- Lavinia confronts her rival…
During the first months of 2025 WWE released several prominent wrestlers who were part of their talent roster. Professional wrestling…
Seventeen year old sprint prodigy Rin Kubo continues to make athletic history in Japan. At the Shizuoka International Athletics Meet,…
This website uses cookies.
Read More