(C) TheJakarta
Last updated on May 23rd, 2022 at 11:21 am


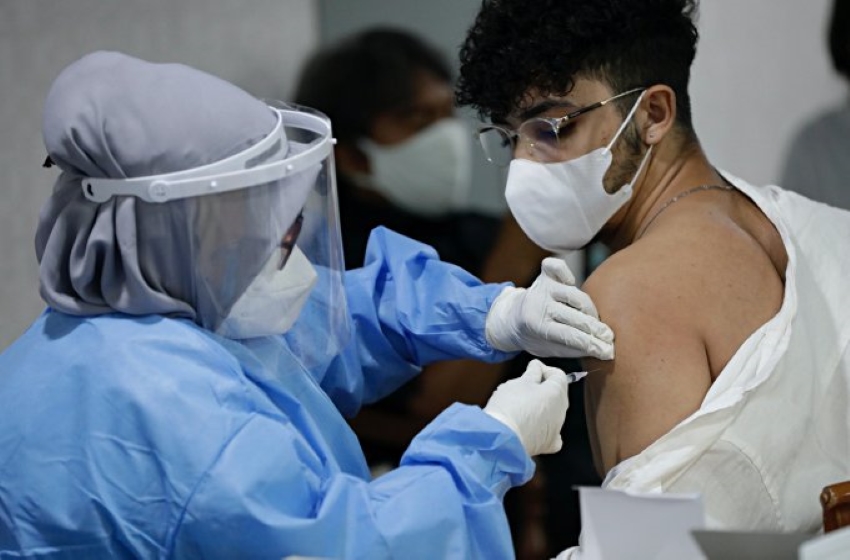
In view of this, Clinical preliminaries will run by plan. Presently, they are getting ready for stage 3. Along with this, Husada’s side is setting up a few angles connected with the clinical preliminary, including members getting antibody infusions, immunizations, specialists, financial plan, and the preparation of the medical clinics answerable for the punches.
The stage three clinical preliminaries will concentrate on insusceptibility to the infection that causes COVID-19 from a bigger number of members than the stage two. An expected 3,500 members will be engaged with the stage 3 clinical preliminaries and will be checked for 6 a year.
Not only that, Husada said that his side had not gathered 3,500 members yet, so endeavors were being made to arrive at that figure. In the mean time, the consequences of the stage 2 clinical preliminary concentrated on the two parts of security and advantages. As far as security, the Merah Putih immunization is demonstrated safe, which has been demonstrated all through clinical preliminaries.
In the interim, from the advantages perspective, Husada said that stage 2 clinical preliminaries showed that invulnerability was made true to form in the antibody beneficiaries.
The Unair Merah Putih Vaccine was created in a joint effort with PT Biotis Pharmaceuticals Indonesia and the Regional General Hospital (RSUD) Dr Soetomo in Surabaya, East Java. Consequently, Stage 1 clinical preliminaries of the constricted or killed infection based COVID-19 antibody had started since February 9, 2022, by infusing the immunization to 90 individuals at the Dr Soetomo Hospital.
Morever, PT Biotis will be liable for the equal creation of the Merah Putih Vaccine during the Phase 3 clinical preliminaries by expanding the size of creation, including of marketed antibodies. Assuming the whole second and third period of the clinical preliminary returns well, the Merah Putih Vaccine will get Emergency Use Authorization (EUA) and be fit to be dispersed to the general population.
OpenAI updated ChatGPT-4o to include its best text-to-image tools so free users can generate Studio Ghibli artwork by giving basic…
The stepping down of Piyush Gupta from the post of CEO of DBS Bank came after 15 years of leading…
The Delhi Directorate of Education releases 2025-26 marks for year-end tests in school levels 6 through 11. Online test data…
Singapore will further cement its status as an important basketball destination when it hosts three FIBA 3x3 events in 2026…
Jewel Section E, directed by Theodore Boborol and starring Ashtine Olviga as Jay-Jay Mariano, Andres Muhlach as Mark Keifer Watson,…
Cebu Pacific celebrates the delivery of its very first aircraft for 2025, the 459-seat Airbus A330neo, delivered at Ninoy Aquino…
This website uses cookies.
Read More