(c) The New York Times
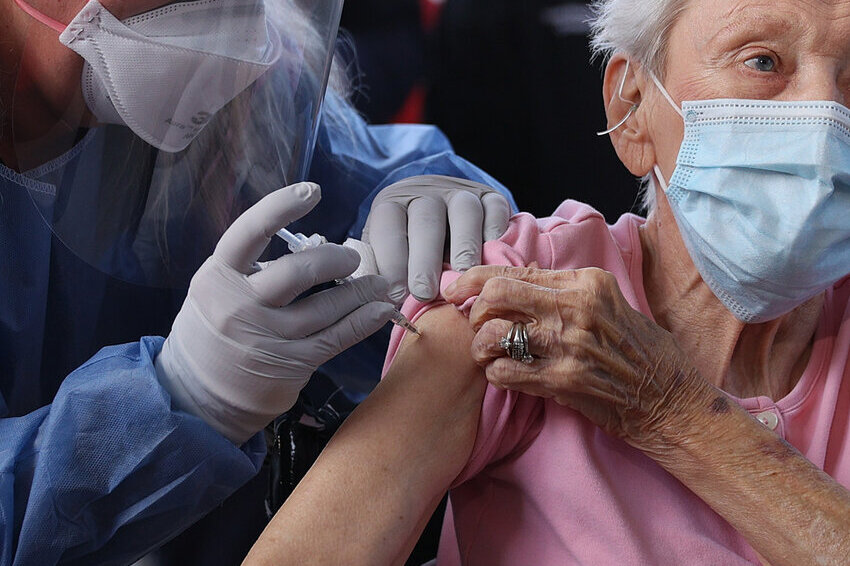
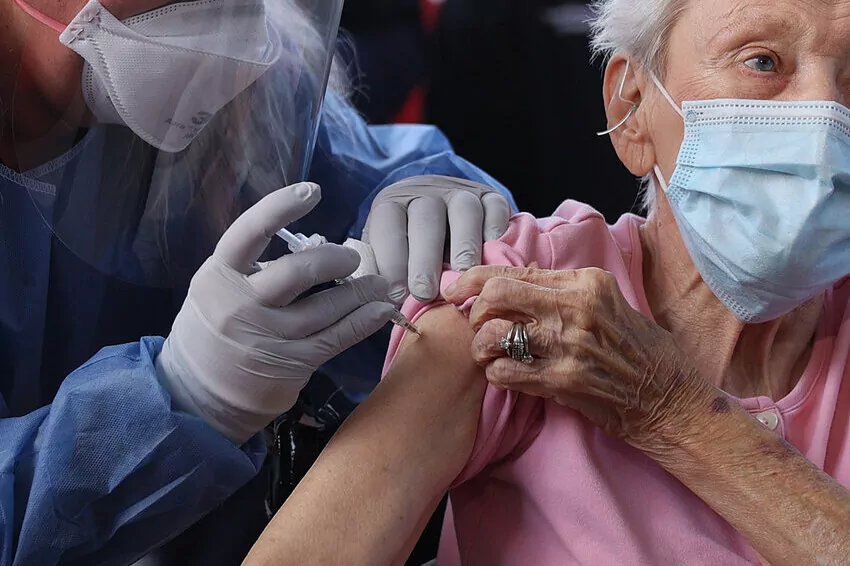
US federal health experts ruled on Friday (Jan 13) that a relationship between Pfizer’s bivalent COVID-19 vaccine and an elevated risk of strokes in those aged 65 and older is “very implausible” and not supported by extensive examination.
A preliminary safety issue found by one of the Centers for Disease Control and Prevention’s (CDC) monitoring systems prompted the CDC to begin an inquiry. Late in November, according to The Washington Post, the problem was initially identified.
Based on early data analyzed by US health regulators, the safety monitoring system has signaled that the revised COVID-19 vaccine may be associated with a form of cerebral stroke in older persons.
On Friday, the US Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) announced that a CDC vaccine database revealed a possible safety issue in which people 65 and older were more likely to suffer an ischemic stroke 21 days after receiving the Pfizer-BioNTech bivalent vaccine, compared to days 22-44.
Brain ischemia, commonly known as an ischemic stroke, is caused by blockages in the arteries that provide blood to the brain.
The FDA and CDC stated that other big trials, the CDC’s Vaccine Adverse Event Reporting System, other nations’ databases, and Pfizer-databases BioNTech’s did not identify this safety concern, adding that further study is required.
The health authorities stated, “Although the existing data shows that it is extremely improbable that the signal in VSD (Vaccine Safety Datalink) reflects an actual clinical risk, we feel it is necessary to convey this information with the public.”
Pfizer and BioNTech stated in a joint statement that they are aware of a small number of reports of ischemic strokes in individuals aged 65 and older after immunization with their revised vaccine.
The companies added, “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the United States and internationally, and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
This safety issue has not been detected with Moderna’s bivalent vaccine, and the CDC and FDA continue to recommend that all individuals aged 6 months and older maintain a current COVID-19 immunization.
Since yesterday, May 2, 2023, at the Mall of Asia Arena in Pasay, Ahtisa Manalo has demonstrated her brilliance by…
“you’re nothing but a trying hard copycat” Character- Lavinia Arguelles Film- Bituing Walang Ningning (1985) Context- Lavinia confronts her rival…
During the first months of 2025 WWE released several prominent wrestlers who were part of their talent roster. Professional wrestling…
Seventeen year old sprint prodigy Rin Kubo continues to make athletic history in Japan. At the Shizuoka International Athletics Meet,…
NextRise 2025-the biggest startup and tech event in Asia-is ready to take place in Seoul on June 26-27 at COEX,…
On this reunion occasion marking 20 years after their debut, the anticipation of the fans seems to be reaching greater…
This website uses cookies.
Read More