(c) CBC
On Wednesday (September 14), the Health Sciences Authority (HSA) gave interim permission for the first bivalent COVID-19 booster vaccination to be used in the country.
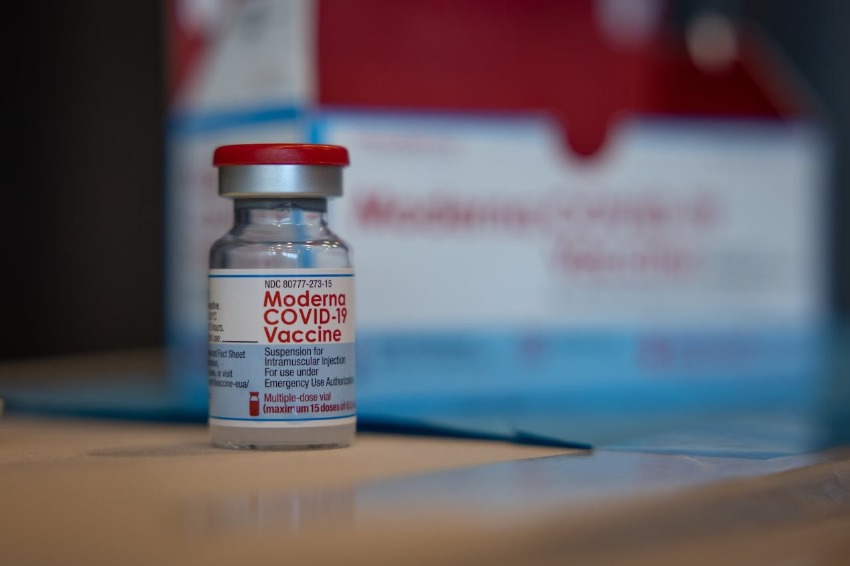
The SARS-CoV-2 original strain and the Omicron BA.1 variant are the ones that are targeted by the two different components of the Spikevax Bivalent Original/Omicron COVID-19 injection that are manufactured by Moderna.
According to a statement made by HSA in a news release, it is an improved version of the Moderna COVID-19 vaccine that is only based on the primary SARS-CoV-2 strain.
Those patients aged 18 years and older who have previously been vaccinated against COVID-19 as part of the primary series are eligible to get it as a booster shot.
The provisional authorization was issued in accordance with the Pandemic Special Access Route in Singapore (PSAR).
According to statements made by Health Minister Ong Ye Kung in front of Parliament on Tuesday, the Ministry of Health has reached agreements with the pharmaceutical companies Pfizer-BioNTech and Moderna to deliver their new bivalent vaccinations into the country.
Both the ancestral strain of COVID-19 and the Omicron variation, which includes the BA.4 and BA.5 strains, are targeted by the bivalent vaccinations.
The Moderna booster shot is a single dosage that consists of two components: 25 micrograms that target the original strain of SARS-CoV-2, and 25 micrograms that target the Omicron BA.1 variant.
In due time, the Expert Committee on COVID-19 Immunization and the Ministry of Health (MOH) will release official vaccination recommendations utilizing this booster, according to the HSA.
HSA stated that it had carefully examined the data from Moderna’s pre-clinical studies, clinical trials in human volunteers, manufacturing and quality controls, and reached the conclusion that the benefits of using the bivalent vaccine as a booster to protect against COVID-19 outweighed the risks of doing so. This was due to the fact that the virus is continuing to evolve.
In addition, the HSA stated that it engaged with members of its Medicines Advisory Committee and Panel of Infectious Diseases Experts before making its conclusion about the regulatory framework.
The regulatory body stated that its clinical analysis was based on an ongoing Phase 2/3 trial that was carried out by Moderna in people who were at least 18 years old.
Chinese President Xi Jinping invited 40 foreign business executives to Beijing on Friday to boost investor confidence and restore stable…
Thailand is set to host Group B of the AFC Women’s Asian Cup 2026 Qualifiers where India, Mongolia, Timor Leste…
The unveiling of an instrument that may symbolize a step toward updating the country’s financial identity is the celebration of…
Big investment company KKR is close to finishing its purchase of Japanese medical equipment manufacturer Topcon as private equity firms…
Indeed, it is, and it marks a milestone in medical research as it transpired that doctors in China successfully transplanted…
Many fans and industry professionals saw Prithviraj Sukumaran's L2 Empuraan movie release on Thursday as a mixed success that gained…
This website uses cookies.
Read More